Popular Design for Metamizole Sodium Injection For Veterinary Drug - Cefquinome Sulfate Injection – Jizhong
Popular Design for Metamizole Sodium Injection For Veterinary Drug - Cefquinome Sulfate Injection – Jizhong Detail:
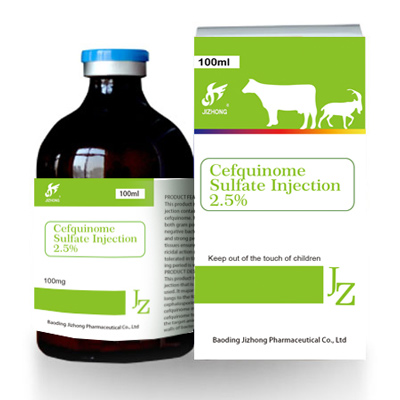
Cefquinome sulfate injection 2.5%
product features:
this product is a kind of suspension for injection containing 25mg/ml of
cefquinome. it is rather potent against both gram positive bacteria and gram
negative bacteria. its features in fast acting and strong penetration through the
tissues ensure the fast and effective bactericidal action of this product. it is well
tolerated in tissues and the drug-withholding period is very short.
product description:
this product is a kind of suspension for injection that is very convenient to be
used. it major ingredient, cefquinome, belongs to the fourth generation of
cephalosporins. the molecular structure of cefquinome makes it very easy for
cefquinome to be quickly distributed in the target animal and penetrate the cell
walls of bacteria. this ensures its fast bactericidal action after it is injected.
cefquinome has a broad spectrum antibacterial activity. it is active against
both gram positive and gram negative bacteria, including actinobacillus,
hemophilus, asteurella, e. coli, staphylococci, streptococci, salmonella
bacteria, clostridium, corynebacteria and erysipelothrix rhusiopathiae. it is also
sensitive to β-lactamase producing bacteria.
major ingredient and its content:
this product contains 25mg/ml of cefquinome.
indications:
it is indicated for the treatment of all kinds of infections caused
by the sensitive bacteria of cefquinome, including respiratory diseases caused
by asteurella, hemophilus, actinobacillus pleuropneumonia and streptococci,
uteritis, mastitis and post partum hypogalactia caused by e.coli and
staphylococci, meningitis caused by staphylococci in pigs, and epidermatitis
caused by staphylococci.
applicable animals: cattle, sheep and pigs
administration and dosage: it shall be administered through
intramuscular injections in a dosage (2mg/kg of body weight calculated as
cefquinome) of 2ml/kg of body weight for piglets and 2ml/kg of body weight for
sows once a day for 2 to 5 successive days.
contraindications: this product is contraindicated in animals or fowls
sensitive to β-lactam antibiotics.
precautions: for animals or fowls sensitive to β-lactam antibiotics, avoid
using this product or any skin contacts with this product.
withholding period: 3 days before slaughtering
packaging: 50ml or 100ml
storage:
it shall be stored under 25℃ shall not be refrigerated.
Product detail pictures:
Related Product Guide:
Well-run equipment, specialist income crew, and better after-sales services; We're also a unified major family, anyone stay with the organization value "unification, determination, tolerance" for Popular Design for Metamizole Sodium Injection For Veterinary Drug - Cefquinome Sulfate Injection – Jizhong , The product will supply to all over the world, such as: Brunei, Istanbul, Guyana, We have advanced production technology, and pursuit innovative in goods. At the same time, the good service has enhanced the good reputation. We believe that as long as you understand our product, you need to be willing to become partners with us. Looking forward to your inquiry.
Factory equipment is advanced in the industry and the product is fine workmanship, moreover the price is very cheap, value for money!