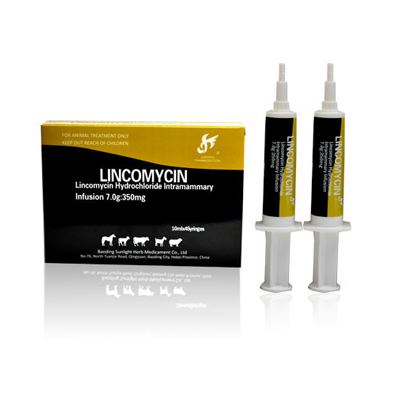
Renewable Design for Ceftiofur Hydrochloride Intramammary Infusion For Veterinary Drug - Lincomycin HCL Intramammary Infusion( Lactating Cow) – Jizhong
Renewable Design for Ceftiofur Hydrochloride Intramammary Infusion For Veterinary Drug - Lincomycin HCL Intramammary Infusion( Lactating Cow) – Jizhong Detail:
Composition:
Each 7.0g Contains:
Iincomycin (as the hydrochloride salt) ……………350mg
Excipient (ad.) ………………………………………….7.0g
Description:
White or almost white oily suspension.
Lincosamide antibiotics. it is mainly used to resist gram-positive bacteria and effect on mycoplasma and some gram-negative bacteria, while has a stronger effect on staphylococcus, streptococcus hemolyticus and pneumococcus. it also has inhibition for anaerobion such as clostridium tetani and bacillus perfringens and is drug-resistant of aerobic gram-negative bacteria. lincomycin is bacteriostat and has bactericidal effect when high concentration. staphylococcus can slowly produce resistant and ha completely cross resistance with clindamycin buthas partial cross resistance with erythromycin.
Indication:
It is used for clinical mastitis and recessive mastitis of cows which caused by sensitive bacteria such as staphylococcus aureu, streptococcus agalactiae, streptococcus dysgalactiae.
Dosage and administration:
Perfuse in milk tube: 1 syringe for each milk area after milking, twice one day, continuously for 2 to 3 days.
Side Effects:
None.
Contraindications:
Do not use in cases of hypersensitivity to lincomycin or to any of the excipients.
Do not use in cases of known resistance to lincomycin.
Withdrawal time:
For meat: 0 day.
For milk: 7 days.
Product detail pictures:
Related Product Guide:
Along with the "Client-Oriented" small business philosophy, a rigorous high-quality handle system, highly developed producing machines and a powerful R&D group, we always supply high-quality products and solutions, fantastic services and aggressive costs for Renewable Design for Ceftiofur Hydrochloride Intramammary Infusion For Veterinary Drug - Lincomycin HCL Intramammary Infusion( Lactating Cow) – Jizhong , The product will supply to all over the world, such as: Philippines, South Africa, Manchester, It is our customers' satisfaction over our products and services that always inspires us to do better in this business. We build mutually beneficial relationship with our clients by giving them large selection of premium car parts at marked down prices. We provide wholesale prices on all our quality parts so you are guaranteed greater savings.
The sales person is professional and responsible, warm and polite, we had a pleasant conversation and no language barriers on communication.